 Infographic: Smashing Crystals View full size JPG | PDF GEORGE RETSECK
Infographic: Smashing Crystals View full size JPG | PDF GEORGE RETSECK
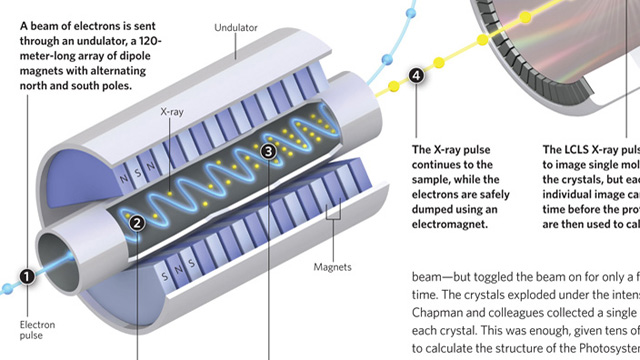
Making high-quality crystals large enough to usefully diffract X-rays is a major headache when attempting to determine protein structures by X-ray crystallography. Researchers prefer crystals that are 100–200 microns in size, with 5 microns being the smallest crystals that can be examined using a synchrotron X-ray source. More powerful X-rays provide better diffraction, but damage the crystals. Henry Chapman, from the Center for Free-Electron Laser Science in Hamburg and colleagues, fed a stream of tiny crystals, as small as 0.2 microns, into an X-ray beam generated by the Linac Coherent Light Source (LCLS)—a billion times more powerful than a synchrotron beam—but toggled the beam on for only a few femtoseconds at a time. The crystals exploded under the intense beam, but not before Chapman and colleagues collected a single diffraction pattern from each crystal. This was enough, given...
| STATS TALK | |||||
| Comparing Stanford'stwo X-ray generating Machines | MINIMUM CRYSTALSIZE viewable by each machine | PULSEDURATION(time neededto capture an image) | NUMBEROF IMAGESgenerated to solve a single structure | NUMBER OF UNDULATORS (housing athousandmagnets) | WAITLIST(once application is accepted) |
| Stanford SynchrotronRadiation Lightsource | 5 µm | 1–10 seconds | 360 | 1 undulatorper X-ray beam | ~1 month |
| Linac CoherentLight Source | 0.2 µm | 2–100 femotoseconds(10-15 of a second) | 3,000,000 | 33 undulators(in a 120 m-long array | >1 year |
Interested in reading more?




