ABOVE: © SCOTT LEIGHTON
During the past year, many medical studies have reported that men are more likely than women to die from COVID-19, the disease caused by SARS-CoV-2. Although disease statistics vary substantially between countries and there are a number of socioeconomic and behavioral factors likely involved, there may be underlying biological mechanisms that also contribute to this imbalance.
Researchers who study sex differences in immunity have discovered several ways in which viral responses differ between people with two X chromosomes and people with one X and one Y (female and male, respectively, for the purposes of this article). Although the mechanisms of SARS-CoV-2 infection are not yet fully understood, it’s possible that some of these sex differences could help explain differences in how infection with the virus affects men and women.
VIRAL ENTRY

SARS-CoV-2 uses the cell membrane proteins ACE2 and TMPRSS2 to enter human cells. Previous research has suggested that ACE2 expression may be downregulated by estrogen, while TMPRSS2 appears to be upregulated by male hormones, or androgens, in some tissues.
VIRAL SENSING/EARLY RESPONSES
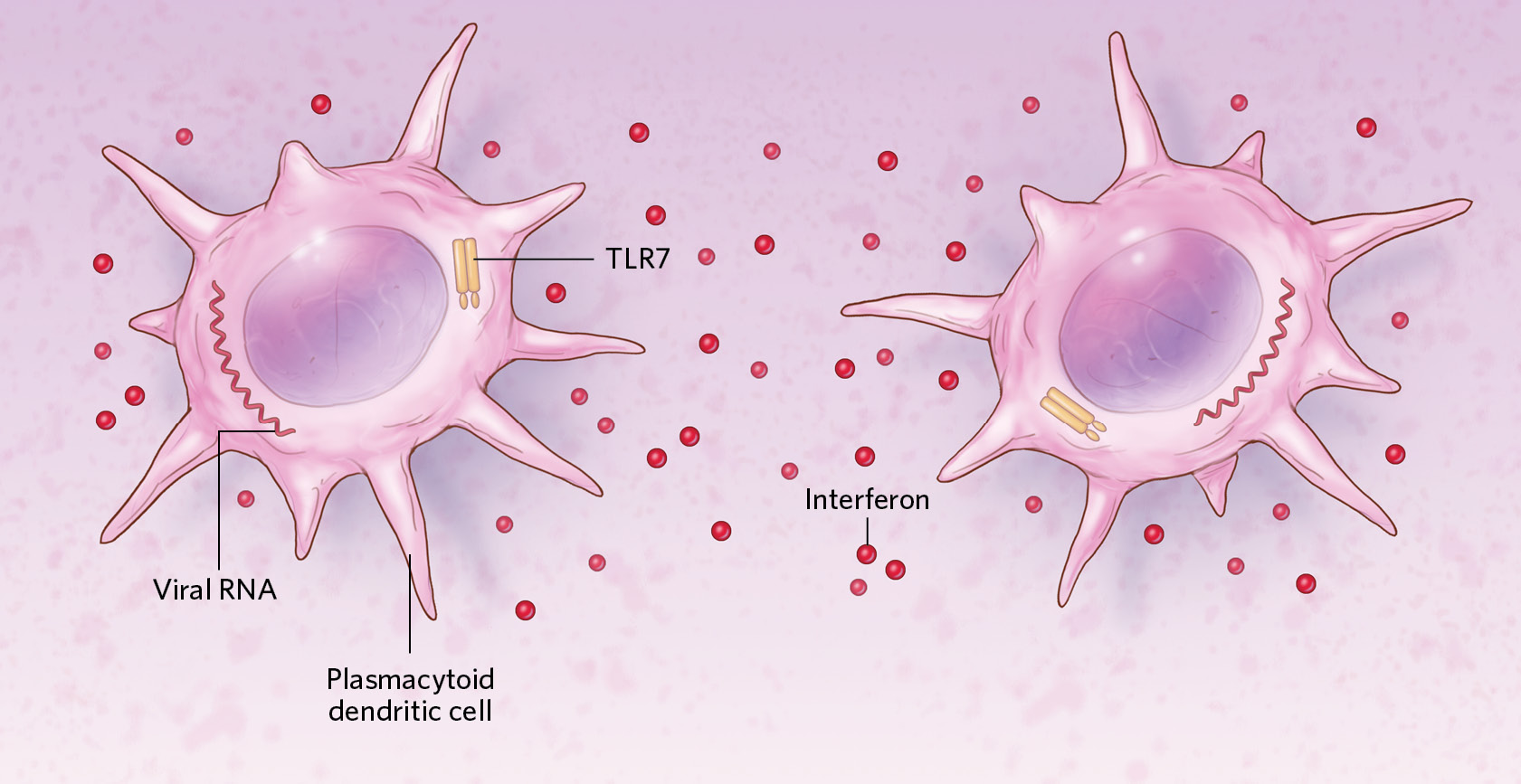
Viral genetic material inside cells can be detected with the help of TLR7, a protein encoded by an X-chromosome gene that escapes normal X inactivation. People with two X chromosomes produce more TLR7 than people with one do. Consistent with this idea, research on other viruses has indicated that TLR7-producing immune cells called plasmacytoid dendritic cells (pDCs) with two X chromosomes tend to release higher quantities of antiviral proteins called interferons than do cells with one X upon infection. In addition, the activity of pDCs and other immune cells may be upregulated by female sex hormones such as estrogen.
INNATE IMMUNITY

Innate immune cells such as neutrophils show higher activation in females than in males in response to some viral infections. These cells can be regulated by sex hormones and may have a more mature phenotype—one that is better prepared to respond to antiviral pathways—in females compared to males. Other immune cells, such as natural killer cells and macrophages, also show differential gene expression depending on sex, perhaps helping to explain the stronger immune responses often seen in females.
ADAPTIVE IMMUNITY
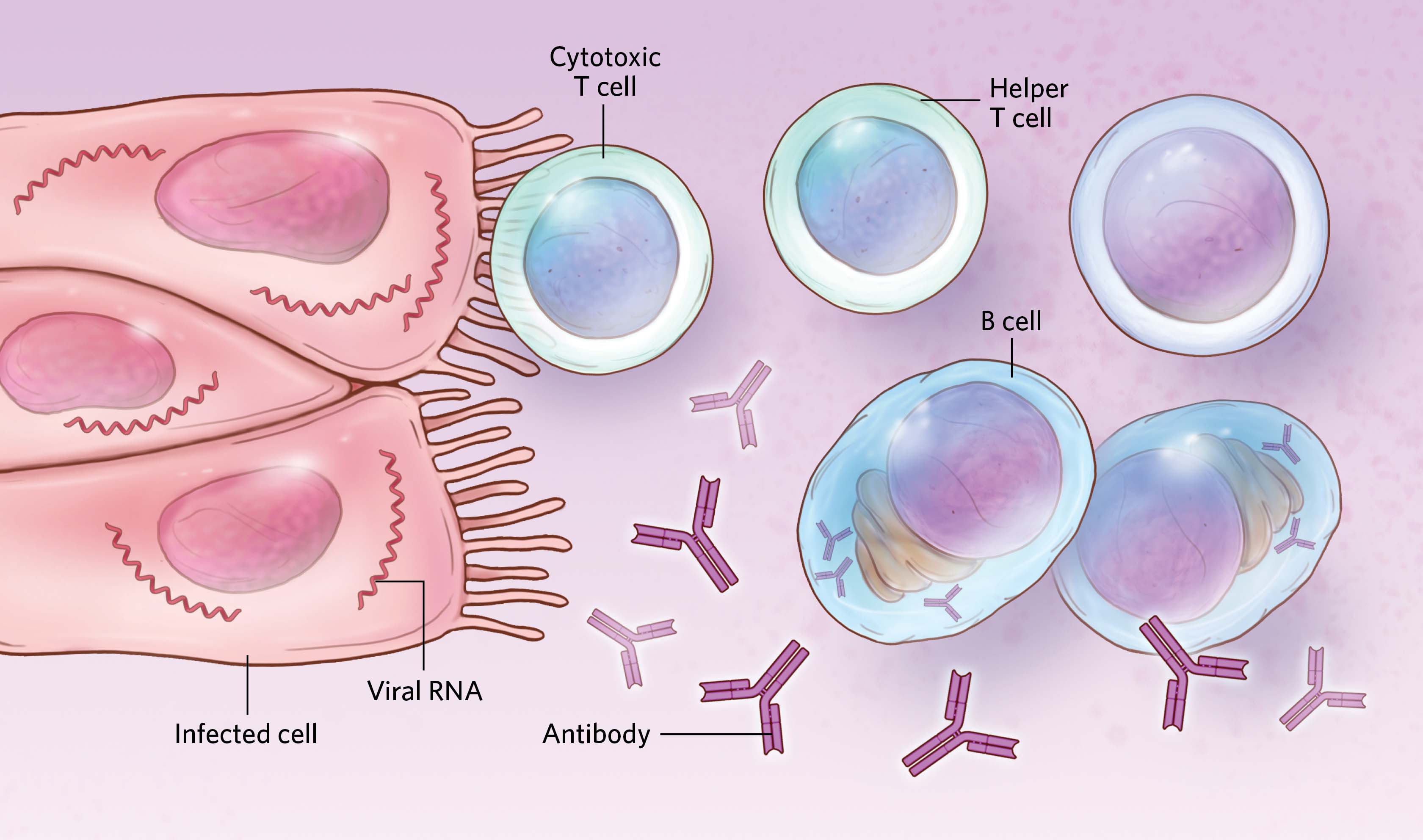
Females have higher numbers and/or higher activity of some types of T cells, which can help trigger adaptive immune responses to viral infections. Studies have also shown that antibody production tends to be lower in males than in females for a number of viral infections and vaccines, although data on sex differences in antibody responses to SARS-CoV-2 are inconsistent. The gene expression and development of cells involved in these responses is likely influenced by sex hormones such as estrogens and androgens.
Read the full story.








