ABOVE: © iStock.com, Ilya Lukichev
Unlike most cells, mature neurons don’t divide. Because mutations often arise through error-prone cell division mechanisms, researchers once believed that these neurons couldn’t accumulate many mutations. Recently, however, scientists discovered that genotoxic stress—which causes DNA damage—can induce somatic mutations in brain cells, and they’re now trying to link the phenomenon to neurodegenerative diseases.
One such neuroscientist, Xiao-Hong Lu from Louisiana State University Health Shreveport, found that many existing tools for probing DNA damage rely on cell replication pathways not present in neurons. “We cannot directly use those methods,” he says, so he made his own, “a sensor specifically for the brain.”
Lu’s genetic sensor, called PRISM (Probe with a viRal proxy for the Instability of DNA surveillance/repair in Somatic brain Mosaicism), uses a viral vector to flag cells that have initiated their DNA damage response (DDR), a set of signaling cascades that regulate DNA repair and cell-cycle checkpoints following genotoxic stress. In many cells, the DDR doesn’t just recognize double-strand DNA breaks, a common form of DNA damage; it also acts as a form of innate immunity, possibly because viruses break DNA to integrate into host genomes. The PRISM viral vector enters many neurons but replicates more readily within cells whose DDR is already activated and therefore unavailable to confront the virus. PRISM’s genome is engineered to include a repeat sequence with a frameshift mutation. Once inside a cell, the host’s DNA replication machinery reverts the frameshift while converting the single-strand DNA to a double-strand one, triggering the production of a fluorescent protein that allows researchers to monitor PRISM-infected neurons in vivo.
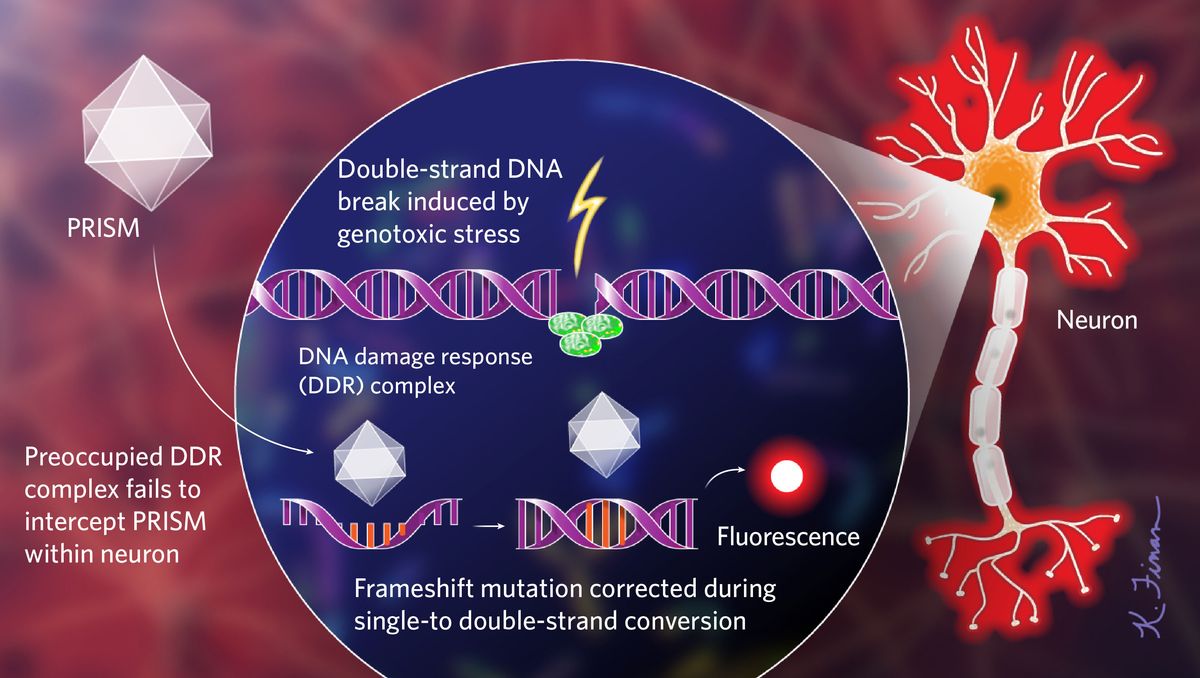
The researchers tested PRISM in mice exposed to paraquat, an herbicide linked to increased Parkinson’s disease (PD) risk, and in a humanized mouse model of a nonhereditary form of PD characterized by the aggregation of misfolded α-synuclein protein. The resulting fluorescence reliably flagged cells that activated their DDR pathways, and those cells continued to fluoresce even after paraquat had been removed or α-synuclein levels reduced. In both experimental mouse populations, PRISM flagged damage in PD’s most affected cell type, dopaminergic neurons in the substantia nigra. Lu analyzed the postmortem brains of six sporadic PD patients, finding that up to 40 percent of the nigral dopaminergic neurons had double-strand breaks— a result he calls “quite prominent.”
Diane Shao, a physician-scientist at Harvard Medical School and Boston Children’s Hospital who was not involved in the study, notes that PRISM is an error-prone but “elegant” tool, saying that “overall, this system is very simple” to apply. She adds, however, that the marker only tags double-strand DNA breaks, which “is only one type in the scheme of DNA damage.”
Lu tells The Scientist that PRISM has applications beyond disease research. The team recently began a NASA-funded project to probe what happens to mice exposed to simulated space radiation, for example. “We know the cells have DNA damage,” he says. “And now we can follow the fate of the cells for a very long time.”








